FDA Approves Xeomin for Comprehensive Upper Facial Wrinkle Treatment
| ABCS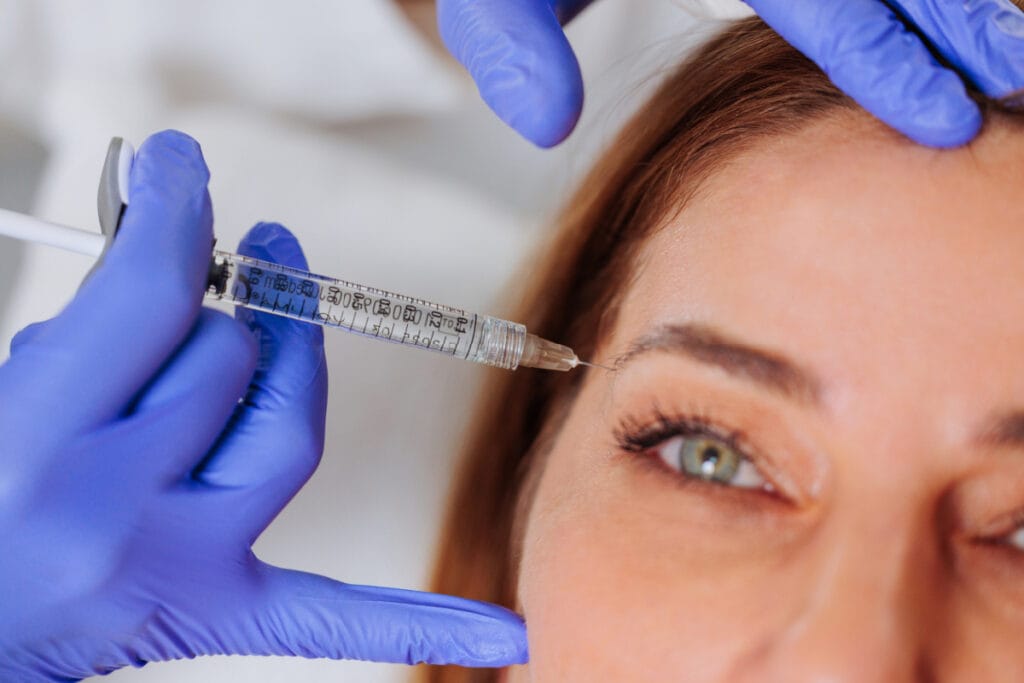
The world of aesthetic treatments has exciting news: Xeomin®, a popular neuromodulator, has just received FDA approval for expanded use. The FDA recently approved Xeomin for the treatment of two major types of upper facial lines: forehead lines and crow’s feet (canthal lines). In addition to its existing approval for treating frown lines (also known as 11s, or glabellar lines), this means that Xeomin is fully FDA-approved for treating wrinkles throughout the upper face.
What is Xeomin?

Xeomin, also known by its scientific name incobotulinumtoxinA, is a neuromodulator used to temporarily relax muscles, reducing the appearance of fine lines and wrinkles. It works similarly to the other popular neuromodulators—Botox®, Dysport®, Daxxify®, and Jeuveau®—but is known for its simplified formula, which contains no accessory proteins. This formulation may reduce the risk of developing resistance, which can lead to reduced results from neuromodulator injections over time.
What does this new FDA approval mean?
This is the first time a neuromodulator other than Botox® has been approved for treating all three cosmetic areas, though the other neuromodulators have all been approved for cosmetic use in treating the glabella.
This will not likely change how the neuromodulators are used or change which neuromodulator your cosmetic surgeon uses, although the FDA approval does specify recommended dosages for each area treated with Xeomin: 20 units for glabellar lines (frown lines), 20 units for forehead lines, and 24 units for canthal lines (crow’s feet).
All 5 FDA-approved neuromodulators are regularly used in all 3 areas for which Xeomin now holds official approval, despite the formulas’ lack of official FDA approval for these areas; this usage is called “off-label” use.
Neuromodulators and Off-Label Use
While this FDA approval is noteworthy, it’s important to understand that all neuromodulators—Botox, Dysport, Jeuveau, and Xeomin—are frequently used “off-label” for forehead lines, crow’s feet, and the glabellar lines. This means that, although the FDA may not have specifically approved these uses, injections in these areas are commonly and safely performed by experienced, qualified providers.
Key Benefits for Patients
The new FDA approval of Xeomin means that research supports its use for a more comprehensive approach to upper facial rejuvenation. Many patients prefer treating all 3 areas at once, as it can result in a more harmonious, balanced appearance. This may also provide reassurance for those patients who prefer Xeomin due to its minimalistic formula, which is thought to be less likely to cause immunity to the formula, a phenomenon dubbed “Botox resistance.”
If you’re considering neuromodulator treatments, consult with a board-certified cosmetic surgeon to learn more about whether neuromodulators could be an appropriate anti-aging solution for you.